
#Periodic table of elements atomic weight free
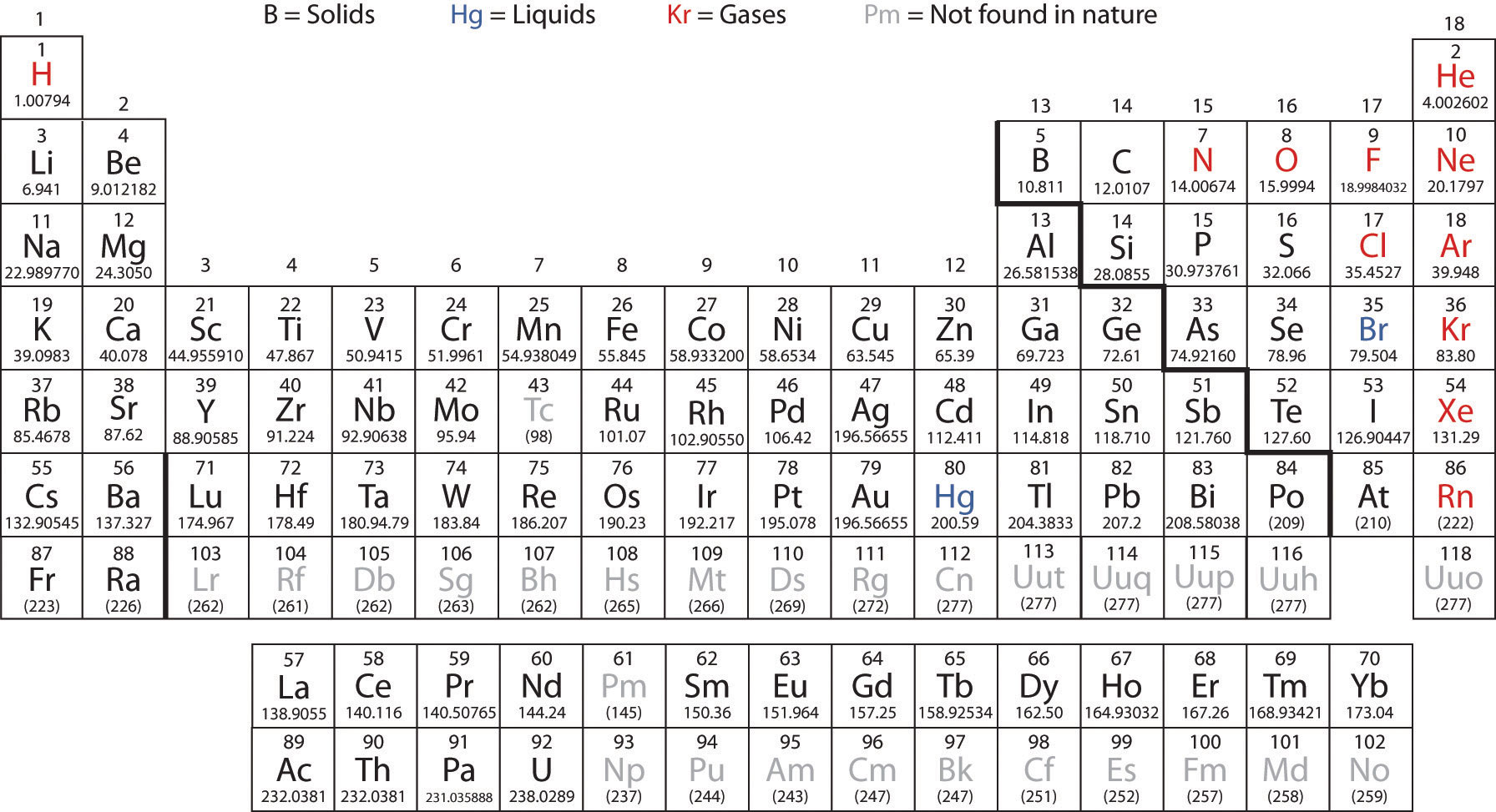
As with Chemistry, the text of Wikipedia is available under the GNU Free Documentation License.The Periodic Table of the Elements H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac#. The list of authors can be seen in the page history. The original article was at List of elements by atomic mass. Series: Alkalis - Alkaline earths - Lanthanides - Actinides - Transition metals - Poor metals - Metalloids - Nonmetals - Halogens - Noble gasesīlocks: s-block - p-block - d-block - f-block - g-block Name | Atomic symbol | Atomic number | Boiling point | Melting point | Density | Atomic mass Standard table | Vertical table | Table with names | Names and atomic masses (large) | Names and atomic masses (small) | Names and atomic masses (text only) | Inline F-block | Elements to 218 | Electron configurations | Metals and nonmetals | Table by blocks | Alternatives Atomic weights of elements with atomic numbers 110-116 taken from this source.

Note 3: The isotopic composition of the element can vary in commercial materials, which can cause the atomic weight to deviate significantly from the given value.Note 2: The isotopic composition of this element varies in some geological specimens, and the variation may exceed the uncertainty stated in the table.However, three elements, Thorium, Protactinium, and Uranium, have a characteristic terrestrial isotopic composition, and thus their atomic mass given. , indicates the mass number of the longest-lived isotope of the element. Note 1: The element does not have any stable nuclides, and a value in brackets, e.g.
#Periodic table of elements atomic weight series
For artificial elements the nucleon count of the most stable isotope is listed bracketsĬhemical series of the periodic table Alkali metals The number in parenthesis gives the uncertainty in the "concise notation" dis given in parenthesis next to the least significant digits to which it applies", e.g., 1.00794(7) stands for 1.00794 ± 0.00007. Each element's atomic number, name, element symbol, and group and period numbers on the periodic table are given. This is a list of chemical elements, sorted by atomic mass (or most stable isotope) and color coded according to type of element.


 0 kommentar(er)
0 kommentar(er)
